Measurement for Concentration
Obtaining concentration of a solution from conductivity
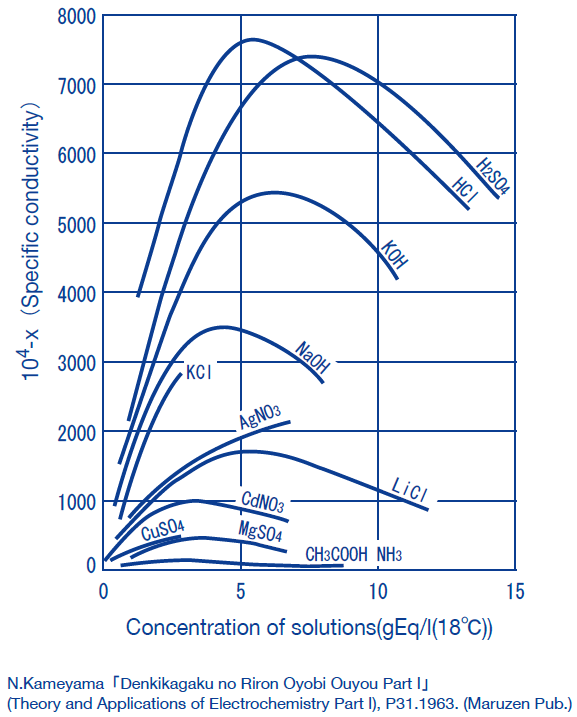
The method of obtaining the concentrations of solutions from conductivity measurements is often used industry.
(Examples:HCl, HNO3, H2SO4, NaCl, NaOH)
In general, the conductivity of electrolyte solutions peak at high concentrations. Note that this means that a single conductivity value corresponds to two concentration values.
Obtaining concentrations by conductivity titration
Let A be the substance to be measured. When a solution (of known concentration) of a substance B that reacts with A is gradually added to a solution of A, A and B react, changing the conductivity of the solution. The reaction will saturate at the point where a certain amount of B has been added (the equivalence point), and following this, as B continues to be added, only the additional B will contribute to the change in conductivity. The amount of A can be obtained from the amount of B that is required to reach the equivalence point. This method is called conductivity titration.
Examples:
Hydrochloric acid (HCl) titrated with sodium hydroxide (NaOH):
H+ + Cl- + Na+ + OH- → Na+ + Cl- + H2O 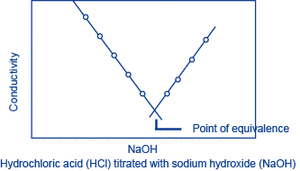
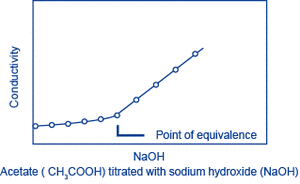
F. W. Fifield and D. Kealey, “Bunsekikagaku I” (Principles and practice of analytical chemistry I), trans. into Japanese, supervised by K. Furuya, Maruzen, 1998, p. 239